A MOLECULAR COMPARISON OF GASES, LIQUIDS, AND SOLIDS
Gases consist of a collection of widely separated particles in constant, chaotic motion. The average energy of the attractions between the particles is much smaller than their average kinetic energy. The lack of strong attractive forces between particles allows a gas to expand to fill its container.
In liquids the intermolecular attractive forces are stronger than in gases and are strong enough to hold particles close together. Thus, liquids are much denser and far less compressible than gases. Unlike gases, liquids have a definite volume, independent of the size and shape of their container. The attractive forces in liquids are not strong enough, however, to keep the particles from moving past one another. Thus, any liquid can be poured, and it assumes the shape of whatever portion of its container it occupies. In solids the intermolecular attractive forces are strong enough to hold particles close together and to lock them virtually in place.
Solids, like liquids, are not very compressible because the particles have little free space between them. Because the particles in a solid or liquid are fairly close together compared with those of a gas, we often refer to solids and liquids as condensed phases. Often the particles of a solid take up positions in a highly regular three-dimensional pattern. Solids that possess highly ordered three- dimensional structures are said to be crystalline. Because the particles of a solid are not free to undergo long- range movement, solids are rigid. Keep in mind, however, that the units that form the solid, whether ions or molecules, possess thermal energy and vibrate in place. This vibrational motion increases in amplitude as a solid is heated. In fact, the energy may increase to the point that the solid either melts or sublimes.
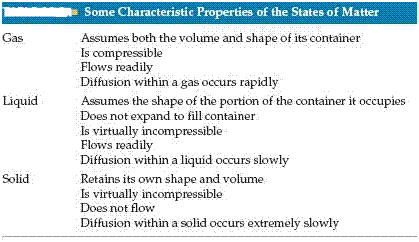
The state of a substance depends largely on the balance between the kinetic energies of the particles and the interparticle energies of attraction. The kinetic energies, which depend on temperature, tend to keep the particles apart and moving. The interparticle attractions tend to draw the particles together.
Substances that are gases at room temperature have weaker interparticle attractions than those that are liquids; substances that are liquids have weaker inter-particle attractions than those that are solids.
The diagram below shows a Molecular-level comparison of gases, liquids, and solids.
INTERMOLECULAR FORCES
The strengths of intermolecular forces of different substances vary over a wide range, but they are generally much weaker than ionic or covalent bonds. Less energy, therefore, is required to vaporize, or evaporate, a liquid or to melt a solid than to break covalent bonds in molecules.
For example, only 16 kJ/mol is required to overcome the intermolecular attractions between HCl molecules in liquid HCl to vaporize it. In contrast, the energy required to break the covalent bond to dissociate HCl into H and Cl atoms is 431 kJ/mol. Thus, when a molecular sub-stance such as HCl changes from solid to liquid to gas, the molecules themselves remain intact.
Many properties of liquids, including their boiling points, reflect the strengths of the intermolecular forces. For example, because the forces between HCl molecules are so weak, HCl boils at a very low temperature, -85 oC at atmospheric pressure.
Ion Dipole Forces
An ion dipole force exists between an ion and the partial charge on the end of a polar molecule. Polar molecules are dipoles; they have a positive end and a negative end. HCl is a polar molecule, for example, because the electronegativities of the H and Cl atoms differ. Positive ions are attracted to the negative end of a dipole, whereas negative ions are attracted to the positive end.
The magnitude of the attraction increases as either the charge of the ion or the magnitude of the dipole moment increases. Ion-dipole forces are especially important for solutions of ionic substances in polar liquids, such as a solution of NaCl in water.
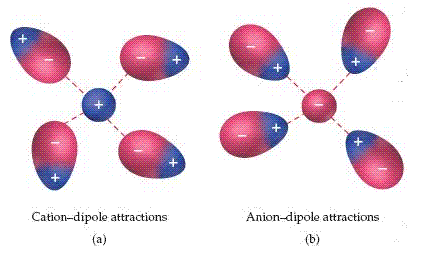
Ion-Dipole Forces at Work
Dipole-Dipole Forces
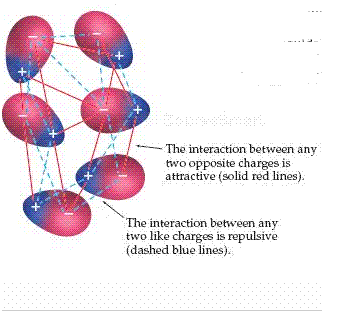
Neutral polar molecules attract each other when the positive end of one molecule is near the negative end of another, as shown in the figure below. These dipole dipole forces are effective only when polar molecules are very close together. Dipole dipole forces are generally weaker than ion dipole forces. In liquids, polar molecules are free to move with respect to one another. As shown in the figure below, the polar molecules will sometimes be in an orientation that is attractive ( red solid lines) and sometimes in an orientation that is repulsive ( blue dashed lines). Two molecules that are attracting each other spend more time near each other than do two that are repelling each other. Thus, the overall effect is a net attraction. When we examine various liquids, we find that for molecules of approximately equal mass and size, the strengths of intermolecular attractions increase with increasing polarity.
*Note that the boiling point increases as the dipole moment increases.
Exercises:
1) 1)An ion-dipole force exists between what?
a. An ion and an ion
b.An ion and a partial charge
c. An ion and a positive charge
d. An ion and a negative charge
2 2) Dipole-dipole forces are ___________________ than ion-dipole forces.
a. Stronger
b. Weaker
c. The same as
d. None of the above
Answers: b; b
Hydrogen Bonding
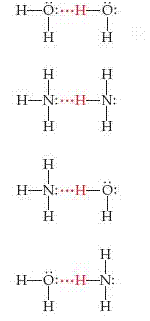
The strong intermolecular attractions in H2O result from hydrogen bonding. Hydrogen bonding is a special type of intermolecular attraction between the hydrogen atom in a polar bond ( particularly an H-F, H-O, or H-N bond) and nonbonding electron pair on a nearby small electronegative ion or atom ( usually an F, O, or N atom in another molecule). For example, a hydrogen bond exists between the H atom in an HF molecule and the F atom of an adjacent HF molecule, F-H...F-H ( where the dots represent the hydrogen bond between the molecules).
Hydrogen bonds can be considered unique dipole dipole attractions. Be-cause F, N, and O are so electronegative, a bond between hydrogen and any of these three elements is quite polar, with hydrogen at the positive end:

The hydrogen atom has no inner core of electrons. Thus, the positive side of the bond dipole has the concentrated charge of the partially exposed, nearly bare proton of the hydrogen nucleus. This positive charge is attracted to the negative charge of an electronegative atom in a nearby molecule. Because the electron-poor hydrogen is so small, it can approach an electronegative atom very closely and thus interact strongly with it.
Examples of Hydrogen bonds:
The energies of hydrogen bonds vary from about 5kJ/mol to 25kJ/mol or so, although there are isolated examples of hydrogen bond energies that are close to 100 kJ/mol. Thus, hydrogen bonds are typically much weaker than ordinary chemical bonds, which have bond energies of 200-1100 kJ/mol. Nevertheless, because hydrogen bonds are generally stronger than dipole-dipole or dispersion forces, they play important roles in many chemical systems, including those of biological significance. For example, hydrogen bonds help stabilize the structures of proteins, which are key parts of skin, muscles, and other structural components of animal tissues.
Densities in Liquid and Solid Phase:

As with most other substances, the solid phase of paraffin is denser than the liquid phase, and the solid therefore sinks below the surface of the liquid paraffin in the beaker on the left. In contrast, the solid phase of water, ice, is less dense than its liquid phase ( right beaker), causing the ice to float on the water.
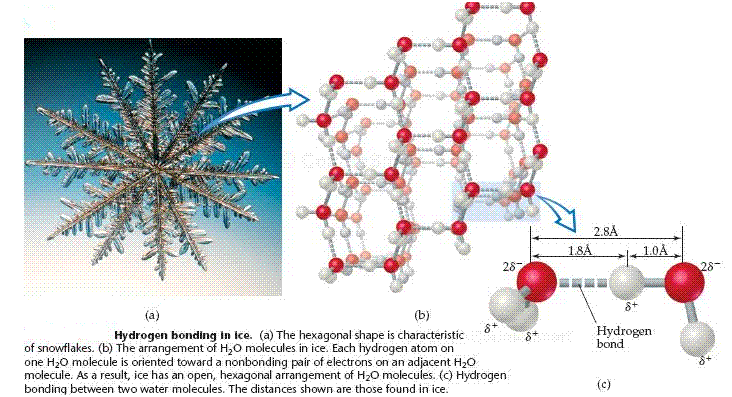
The lower density of ice compared to that of water can be understood in terms of hydrogen- bonding interactions between H2O molecules. In ice, the H2O molecules assume an ordered, open arrangement. This arrangement optimizes the hydrogen bonding interactions between molecules, with each H2O molecule forming hydrogen bonds to four other H2O molecules. These hydrogen bonds, however, create the open cavities shown in the structure. When the ice melts, the motions of the molecules cause the structure to collapse. The hydrogen bonding in the liquid is more random than in ice, but it is strong enough to hold the molecules close together. Consequently, liquid water has a more dense structure than ice, meaning that a given mass of water occupies a smaller volume than the same mass of ice.
Hydrogen Bonding in Ice:
Comparing Intermolecular Forces
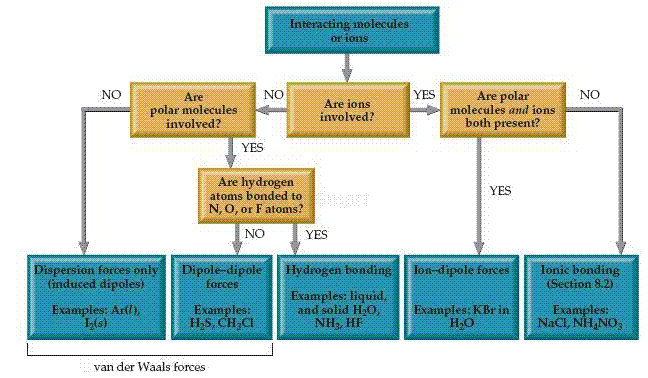
We can identify the intermolecular forces that are operative in a substance by considering its composition and structure. Dispersion forces are found in all sub-stances. The strengths of these attractions increase with increasing molecular weight and depend on molecular shapes. Dipole dipole forces add to the effect of dispersion forces and are found in polar molecules. Hydrogen bonds, which require H atoms bonded to F, O, or N, also add to the effect of dispersion forces. Hydrogen bonds tend to be the strongest type of intermolecular attraction. None of these intermolecular attractions, however, is as strong as ordinary ionic or covalent bonds. In general, the energies associated with the dispersion forces and dipole dipole forces are in the range of , while the energies of hydrogen bonds are in the range . Ion dipole attractions lead to energies of approximately .
Flowchart for determining intermolecular forces. London dispersion forces occur in all instances. The strengths of the other forces generally increase proceeding from left to right across the chart.
Properties Of Liquids
Viscosity
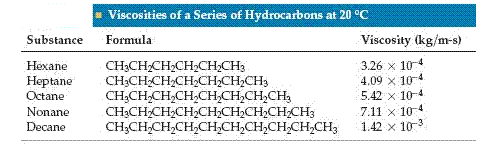
Some liquids, such as molasses and motor oil, flow very slowly; others, such as water and gasoline, flow easily. The resistance of a liquid to flow is called its viscosity. The greater a liquids viscosity, the more slowly it flows. Viscosity can be measured by timing how long it takes a certain amount of the liquid to flow through a thin tube under gravitational force. More viscous liquids take longer. Viscosity can also be determined by measuring the rate at which steel balls fall through the liquid. The balls fall more slowly as the viscosity increases.

Viscosity is related to the ease with which individual molecules of the liquid can move with respect to one another. It thus depends on the attractive forces between molecules, and on whether structural features exist that cause the molecules to become entangled ( for example, long molecules might become tangled like spaghetti). The SI units for viscosity are . For any given substance, viscosity decreases with increasing temperature.
Surface Tension
The surface of water behaves almost as if it had an elastic skin, as evidenced by the ability of certain insects to walk on water. This behavior is due to an imbalance of intermolecular forces at the surface of the liquid. Notice that molecules in the interior are attracted equally in all directions, whereas those at the surface experience a net inward force. The resultant inward force pulls molecules from the surface into the interior, thereby reducing the surface area and making the molecules at the surface pack closely together. Because spheres have the smallest surface area for their volume, water droplets assume an almost spherical shape. Similarly, water tends to "bead up" on a newly waxed car because there is little or no attraction between the polar water molecules and the nonpolar wax molecules.

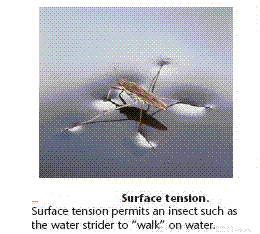
A measure of the inward forces that must be overcome to expand the surface area of a liquid is given by its surface tension. Surface tension is the energy required to increase the surface area of a liquid by a unit amount. For example, the surface tension of water at 20oC is 7.29 x 10-2 J/m2, which means that an energy of 7.29 x 10-2 must be supplied to increase the surface area of a given amount of water by 1m2. Water has a high surface tension because of its strong hydrogen bonds. The surface tension of mercury is even higher because of even stronger metallic bonds between the atoms of mercury.
Exercises:
1) 1) The resistance of a liquid to flow is called
a. Molarity
b. Surface tension
c. Molality
d. Viscosity.
2 2)Why does water have a high surface tension?
a. Strong hydrogen bonds
b. High molal concentration
c. High viscosity
d. Low viscosity
Answers: d; a
Phase Changes
Water left uncovered in a glass for several days evaporates. An ice cube left in a warm room quickly melts. Solid CO2 ( sold as dry ice) sublimes at room temperature; that is, it changes directly from the solid to the vapor state. In general, each state of matter can change into either of the other two states. These transformations are called either phase changes or changes of state.
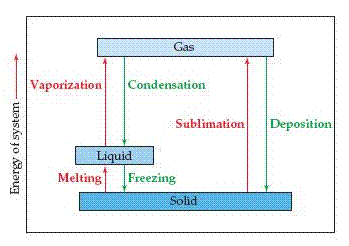
Energy Changes Accompanying Phase Changes
When a solid melts, the units that made up the solid are freed to move with respect to one another, which ordinarily means that their average separations increase. This melting process is called fusion. The increased freedom of motion of the molecules or ions comes at a price, measured by the heat of fusion, or enthalpy of fusion, denoted  . The heat of fusion of ice, for example, is 6.01 kJ/mol.
. The heat of fusion of ice, for example, is 6.01 kJ/mol.
As the temperature of the liquid phase increases, the molecules of the liquid move about with increasing energy. One measure of this increasing energy is that the concentration of gas- phase molecules over the liquid increases with temperature. These molecules exert a pressure called the vapor pressure.
For now we just need to understand that the vapor pressure increases with increasing temperature until it equals the external pressure over the liquid, typically atmospheric pressure. At this point the liquid boils the molecules of the liquid move into the gaseous state, where they are widely separated. The energy required to cause this transition is called the heat of vaporization, or enthalpy of vaporization, denoted 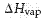 . For water, the heat of vaporization is 40.7 kJ/mol.
. For water, the heat of vaporization is 40.7 kJ/mol.
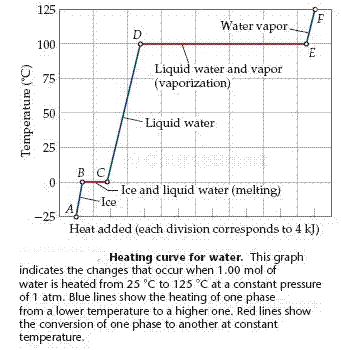
A graph of the temperature of the system versus the amount of heat added is called a heating curve.
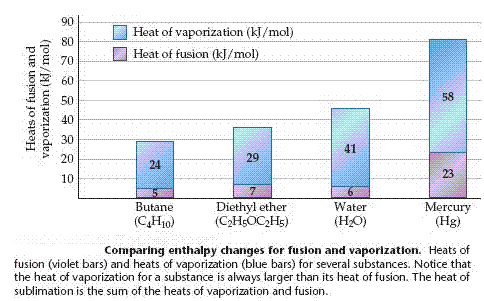
The molecules of a solid can be transformed directly into the gaseous state. The enthalpy change required for this transition is called the heat of sublimation, denoted 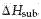 .
. 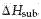 is the sum of
is the sum of  and
and 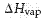 . Thus, for water is approximately 47 kJ/mol.
. Thus, for water is approximately 47 kJ/mol.
Heating Curve of Water
Vapor Pressure on the Molecular Level
Dynamic Equilibrium
The condition in which two opposing processes are occurring simultaneously at equal rates is called a dynamic equilibrium, but is usually referred to merely as equilibrium. A liquid and its vapor are in dynamic equilibrium when evaporation and condensation occur at equal rates. It may appear that nothing is occurring at equilibrium because there is no net change in the system. In fact, a great deal is happening: molecules continuously pass from the liquid state to the gas state and from the gas state to the liquid state. All equilibrium between different states of matter possess this dynamic character. The vapor pressure of a liquid is the pressure exerted by its vapor when the liquid and vapor states are in dynamic equilibrium.
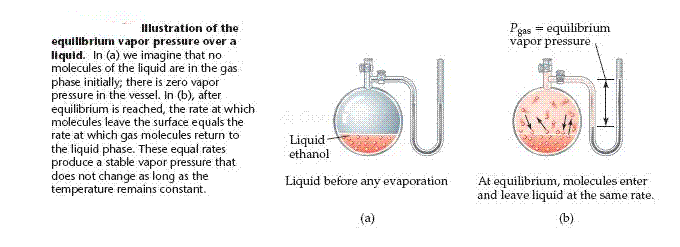
Volatility, Vapor Pressure, and Temperature
When vaporization occurs in an open container, as when water evaporates from a bowl, the vapor spreads away from the liquid. Little, if any, is recaptured at the surface of the liquid. Equilibrium never occurs, and the vapor continues to form until the liquid evaporates to dryness. Substances with high vapor pressure (such as gasoline) evaporate more quickly than substances with low vapor pressure (such as motor oil). Liquids that evaporate readily are said to be volatile.
Vapor Pressure and Boiling Point
A liquid boils when its vapor pressure equals the external pres-sure acting on the surface of the liquid. At this point bubbles of vapor form within the liquid. The temperature at which a given liquid boils increases with increasing external pressure. The boiling point of a liquid at 1 atm (or 760 torr) pressure is called its normal boiling point.
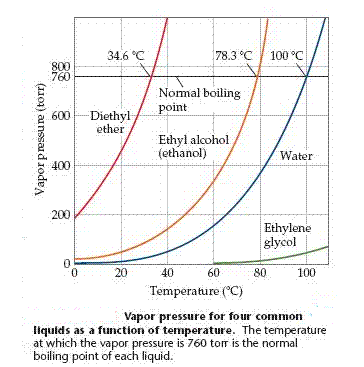
The atmospheric pressure is lower at higher altitudes, so water boils at a lower temperature and foods generally take longer to cook.
PHASE DIAGRAMS
The equilibrium between a liquid and its vapor is not the only dynamic equilibrium that can exist between states of matter. Under appropriate conditions of temperature and pressure, a solid can be in equilibrium with its liquid state or even with its vapor state. A phase diagram is a graphic way to summarize the conditions under which equilibrium exist between the different states of matter. Such a diagram also allows us to predict the phase of a substance that is stable at any given temperature and pressure.
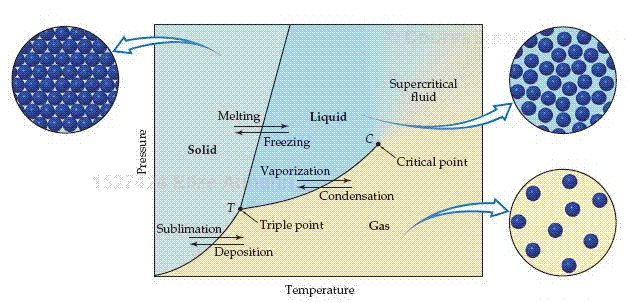
1. The line from T to C is the vapor-pressure curve of the liquid. It represents the equilibrium between the liquid and gas phases. The point on this curve where the vapor pressure is 1 atm is the normal boiling point of the sub-stance. The vapor-pressure curve ends at the critical point ( C), which is at the critical temperature and critical pressure of the substance. Beyond the critical point the liquid and gas phases become indistinguishable from each other, and the state of the substance is a supercritical fluid.
2. The line that separates the solid phase from the gas phase represents the change in the vapor pressure of the solid as it sublimes at different temperatures.
3. The line that separates the solid phase from the liquid phase corresponds to the change in melting point of the solid with increasing pressure. This line usually slopes slightly to the right as pressure increases, because for most substances the solid form is denser than the liquid form. An increase in pressure usually favors the more compact solid phase; thus, higher temperatures are required to melt the solid at higher pressures. The melting point of a substance is identical to its freezing point. The two differ only in the direction from which the phase change is approached. The melting point at 1 atm is the normal melting point.
Point T, where the three curves intersect, is known as the triple point. All three phases are in equilibrium at this temperature and pressure. Any other point on the three curves represents equilibrium between two phases. Any point on the diagram that does not fall on a line corresponds to conditions under which only one phase is present. The gas phase, for example, is stable at low pressures and high temperatures, whereas the solid phase is stable at low temperatures and high pressures. Liquids are stable in the region between the other two.
Exercises:
1) 1) What is a graphic way to summarize the conditions under which equilibrium exist between the different states of matter?
a. Heating curve
b. Equilibrium model
c. Phase diagram
d. All of the above
2 2) The melting point at 1 atm is the:
a. Wrong melting point
b. Normal melting point
c. Lowered melting point
d. Higher melting point
Answers: c; b
Structures of Solids
Solids can be either crystalline or amorphous (noncrystalline). In a crystalline solid the atoms, ions, or molecules are ordered in well-defined three-dimensional arrangements.
In a crystalline solid the atoms, ions, or molecules are ordered in well-defined three- dimensional arrangements.
An amorphous solid (from the Greek words for without form) is a solid in which particles have no orderly structure. These solids lack well-defined faces and shapes. Many amorphous solids are mixtures of particles that do not stack together well. Most others are composed of large, complicated molecules. Familiar amorphous solids include rubber and glass.
Because the particles of an amorphous solid lack any long-range order, intermolecular forces vary in strength throughout a sample. Thus, amorphous solids do not melt at specific temperatures. Instead, they soften over a temperature range as intermolecular forces of various strengths are overcome. A crystalline solid, in contrast, melts at a specific temperature.
BONDING IN SOLIDS
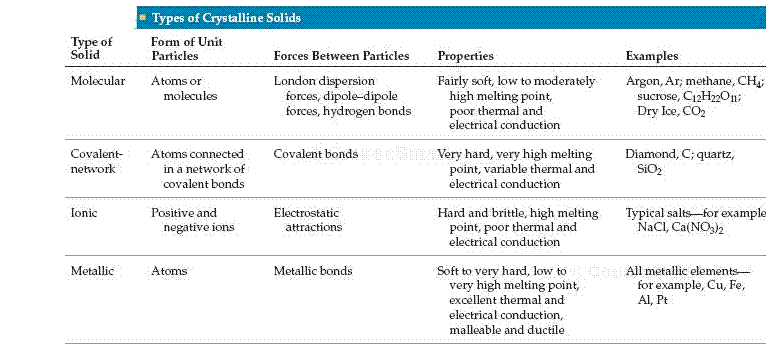
Molecular Solids
Molecular solids consist of atoms or molecules held together by intermolecular forces (dipole-dipole forces, London dispersion forces, and hydrogen bonds). Because these forces are weak, molecular solids are soft. Furthermore, they normally have relatively low melting points (usually below 200 oC). Most sub-stances that are gases or liquids at room temperature form molecular solids at low temperature. Examples include Ar, H2O, and CO2.
Exercise:
1) Name the four types of solids.
Answer: Molecular, covalent-network, ionic, and metallic