Atoms - The Basic Structure
 Atoms are the basis of chemistry. They are the basis for everything in the Universe. We're going to cover basics like atomic structure and bonding between atoms. As you learn about chemistry, remember that atoms form compounds that help the biological world survive.
Atoms are the basis of chemistry. They are the basis for everything in the Universe. We're going to cover basics like atomic structure and bonding between atoms. As you learn about chemistry, remember that atoms form compounds that help the biological world survive.
Q: Which of these choices is not a major part of an atom?
A. Electrons
B. Protons
C. Neutrons
D. All are parts of an atom
A: All of these particles are found in atoms. Hydrogen is a little special because it does not have a neutron, but all of the other elements have varying numbers of electrons, neutrons, and protons. Neutral atoms have the same number of electrons and protons.
SMALLER THAN ATOMS?
Are there pieces of matter that are smaller than atoms? Sure there are. You'll soon be learning that atoms are composed of pieces like neutrons, electrons, and protons. But guess what? There are even smaller particles moving around in atoms. These super-small particles can be found inside the protons and neutrons. Scientists have many names for those pieces, but you may have heard of nucleons and quarks. Nuclear chemists and physicists work together with particle accelerators to discover the presence of these tiny, tiny, tiny pieces of matter.
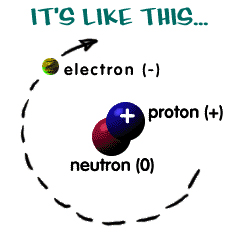
Even though those super tiny atomic particles exist, there are three basic parts of an atom. The parts are the electrons, protons, and neutrons. What are electrons, protons, and neutrons? A picture works best. You have a basic atom. There are three pieces to an atom. There are electrons, protons, and neutrons. That's all you have to remember. Three things! As you know, there are over 100 elements in the periodic table. The thing that makes each of those elements different is the number of electrons, protons, and neutrons. The protons and neutrons are always in the center of the atom. Scientists call the center of the atom the nucleus. The electrons are always found whizzing around the center in areas called orbitals.
Q: Which of these particles is found in the atomic nucleus?
A. Electrons and Protons
B. Neutrons and Electrons
C. Protons and Neutrons
A: Protons and neutrons are both found in the nucleus of an atom. Electrons are very small particles found spinning around the nucleus in different orbitals.
 You can also see that each piece has either a "+", "-", or a "0." That symbol refers to the charge of the particle. You know when you get a shock from a socket, static electricity, or lightning? Those are all different types of electric charges. There are even charges in tiny particles of matter like atoms. The electron always has a "-" or negative charge. The proton always has a "+" or positive charge. If the charge of an entire atom is "0", that means there are equal numbers of positive and negative pieces, equal numbers of electrons and protons. The third particle is the neutron. It has a neutral charge (a charge of zero).
You can also see that each piece has either a "+", "-", or a "0." That symbol refers to the charge of the particle. You know when you get a shock from a socket, static electricity, or lightning? Those are all different types of electric charges. There are even charges in tiny particles of matter like atoms. The electron always has a "-" or negative charge. The proton always has a "+" or positive charge. If the charge of an entire atom is "0", that means there are equal numbers of positive and negative pieces, equal numbers of electrons and protons. The third particle is the neutron. It has a neutral charge (a charge of zero).
Q: The atomic number of an element tells you the number of ___________.
A. Electrons or Neutrons
B. Electrons or Protons
C. Protons or Neutrons
A: The atomic number of an element tells you the number of electrons or protons found in a neutral atom of that element. Ions have varying numbers of electrons and isotopes have varying numbers of neutrons.
adapted from Rader's Chem4kids.com
 Atoms are the basis of chemistry. They are the basis for everything in the Universe. We're going to cover basics like atomic structure and bonding between atoms. As you learn about chemistry, remember that atoms form compounds that help the biological world survive.
Atoms are the basis of chemistry. They are the basis for everything in the Universe. We're going to cover basics like atomic structure and bonding between atoms. As you learn about chemistry, remember that atoms form compounds that help the biological world survive.  You can also see that each piece has either a "+", "-", or a "0." That symbol refers to the charge of the particle. You know when you get a shock from a socket, static electricity, or lightning? Those are all different types of electric charges. There are even charges in tiny particles of matter like atoms. The electron always has a "-" or negative charge. The proton always has a "+" or positive charge. If the charge of an entire atom is "0", that means there are equal numbers of positive and negative pieces, equal numbers of electrons and protons. The third particle is the neutron. It has a neutral charge (a charge of zero).
You can also see that each piece has either a "+", "-", or a "0." That symbol refers to the charge of the particle. You know when you get a shock from a socket, static electricity, or lightning? Those are all different types of electric charges. There are even charges in tiny particles of matter like atoms. The electron always has a "-" or negative charge. The proton always has a "+" or positive charge. If the charge of an entire atom is "0", that means there are equal numbers of positive and negative pieces, equal numbers of electrons and protons. The third particle is the neutron. It has a neutral charge (a charge of zero).